Expanding Inclusivity: Tranexamic Acid for the Treatment of Melasma in Males
4/1/2024
INTRODUCTION
Tranexamic acid (TXA) is an antifibrinolytic medication largely known for its efficacy in managing menorrhagia, or heavy periods, making it a medication predominantly
used by women. It was first used in the 1960s, and by the 1970s, early studies revealed tranexamic acid’s effectiveness in reducing excessive menstrual bleeding.1 TXA is now a standard for treating menorrhagia.
More recently, TXA has found a new dermatologic purpose for the improvement of any melasma-related pigmentation. Melasma is a skin condition commonly characterized by dark patches or irregularities in pigmentation in the skin, particularly in sun-exposed areas.2,3 Subsequent studies have shown promising results in TXA’s effectiveness in reducing melanogenesis , the process by which pigment in the skin and hair is produced.4 Although, many of these studies largely focus on female populations, leaving male melasma patients in the minority. The historical background of the drug’s initial use could largely be the reason why there are so few male patients included in current studies. Melasma can negatively impact an individual’s self-esteem and confidence thereby reducing the quality of life of an individual.5 We hope to shine a light on the efficacious nature of oral TXA on male melasma patients. We now consider oral TXA for all melasma patients, male and female, before undergoing any additional treatments with lasers or chemical peels.
CASES
&srotate=0)
CASE 1
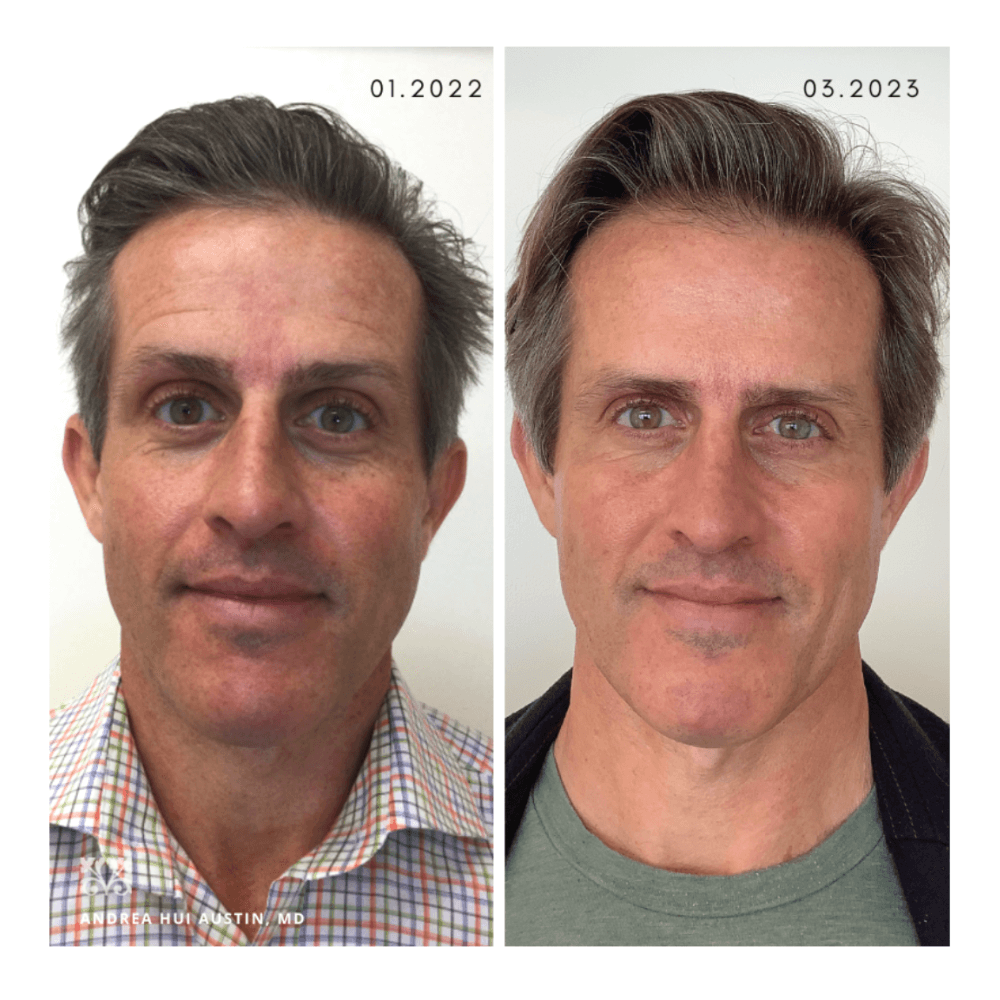
Case 1:
This is a 45-year-old male with moderate melasma on his face and neck. He was started on a regimen of oral tranexamic acid 650 mg daily, along with topical tretinoin 0.05% gel every evening and topical tranexamic acid 3% serum (Discoloration Defense by Skinceuticals, New York, NY) twice daily, along with daily physical sunblock. He received four treatments of KTP vascular laser (ExcelV by Cutera, Brisbane, CA). Almost complete resolution was achieved about 14 months from the first appointment. He has maintained his results with the same oral and topical regimen as of publication.
&srotate=0)
CASE 2
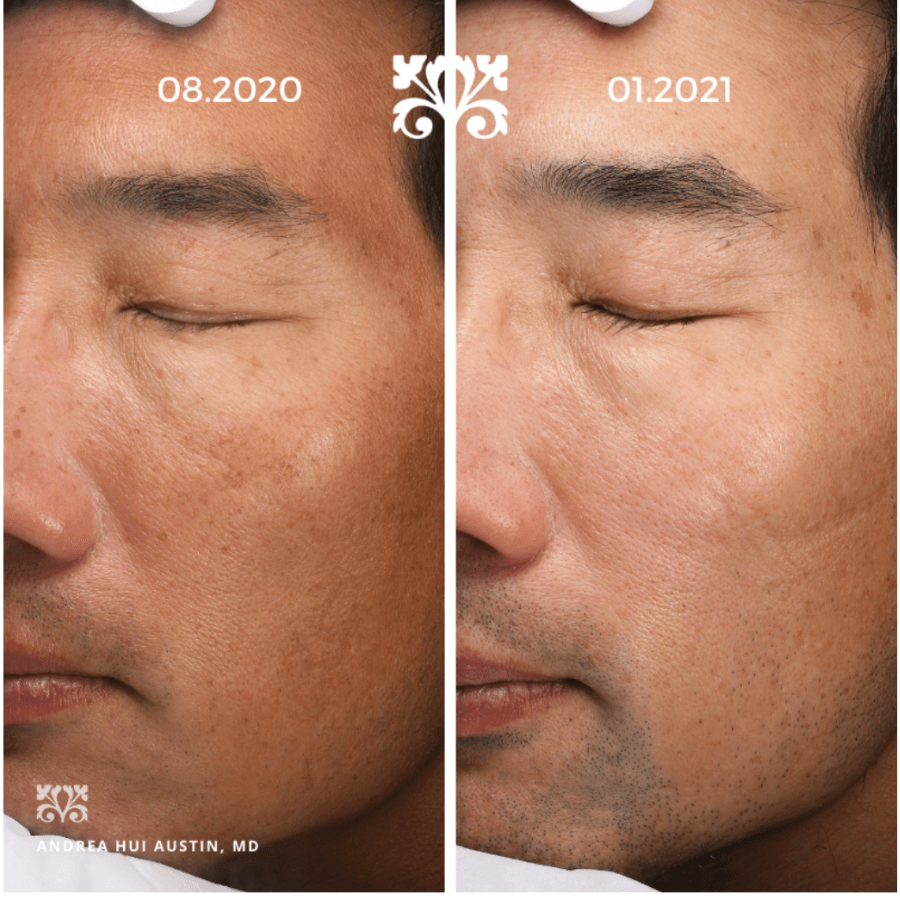
Case 2:
This is a 55-year-old man with moderate melasma and also a self-described outdoorsman and surfer. He was often tanned and not a candidate for laser. He was started on a regimen of oral tranexamic acid 650 mg daily and topical compounded tretinoin 0.025%/hydroquinone 4%/hydrocortisone 0.5%/kojic acid 6% by Sincerus Pharmaceuticals, Pompano Beach, FL) every evening. He received a series of 6 chemical peels (Vitalize peel by Allergan, Irvine, CA) with complete resolution of his melasma.
&srotate=0)
CASE 3
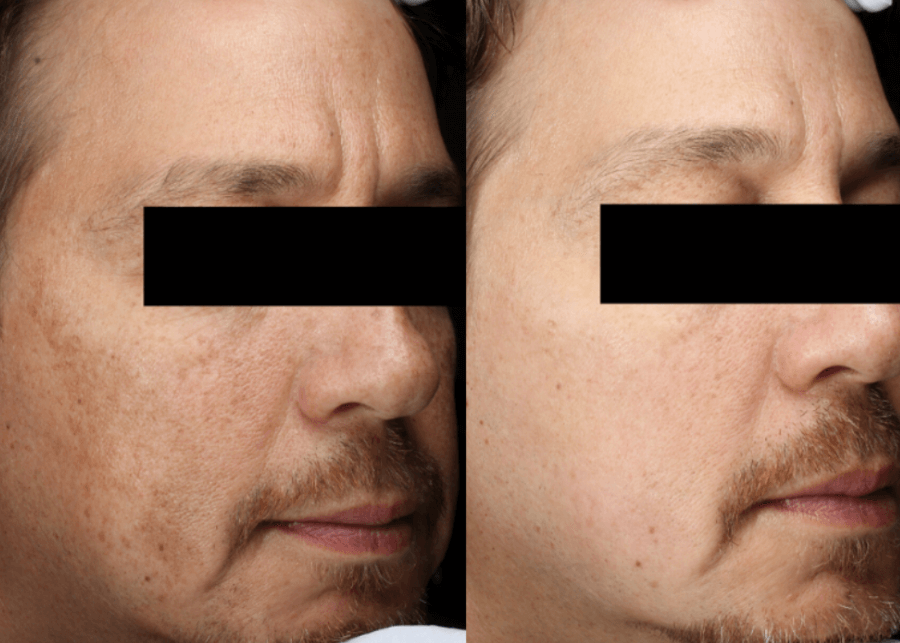
Case 3:
This 56-year-old gentleman had a combination of severe melasma superimposed with sun damage. He began a regimen of oral tranexamic acid 650 mg daily, along with topical retinol 1% (AlphaRet by SkinBetter Science, Phoenix, AZ) every evening along with physical sunblock daily. He received two chemical peels (Vitalize peel by Allergan, Irvine, CA) and two treatments of Q-switched Alexandrite laser (Alex Trivantage by Candela, Marlborough, MA) with near-complete resolution. He has maintained his results with the same oral and topical regimen as of publication.
DISCUSSION
Melasma is a common, acquired skin condition that can be caused by a multitude of factors including genetics, radiation, hormones,cosmetics,andphototoxicdrugusage.6 TXAcanbe administered for all severities of melasma.The exact mechanism for how TXA reduces melanogenesis is not yet fully understood. What we believe is a probable explanation for its effectiveness is TXA’s ability to inhibit the activity of plasmin. Plasmin can stimulate melanocytes, the cells that are responsible for producing melanin. By inhibiting plasmin, TXA can reduce the stimulation of melanocytes thus decreasing melanin synthesis.7 TXA may also be involved with the interference of melanin synthesis. TXA is structurally similar to tyrosinase, an enzyme that plays a crucial role in melanin production and may work by competitively antagonizing its function.3 Additionally, it has been found that TXA has anti-inflammatory properties and can modulate the inflammatory response by influencing cytokine levels.8 Normally, chronic inflammation can trigger melanogenesis and contribute to hyperpigmentation. Thereby, with the reduction of this inflammation, TXA can indirectly inhibit melanin production. While more research is needed to fully understand the exact mechanisms behind TXA’s dermatologic effectiveness, TXA has brought great success in the many melasma patients we have seen.
There are a few studies in the current literature that support the use of TXA in treating melasma, although currently, there are no studies that only focus on men. Even so, when studies do include a male population, few males make up a portion of the sample size. In one of the largest studies of oral TXA, 561 patients were enrolled, while only 8.6% of those patients were male. The majority of patients found an improvement in their melasma symptoms.9 A randomized controlled trial of 96 patients compared the effect of TXA on intraoperative blood loss (IOB) based on gender. Interestingly, it was found that there was an effect in women, but none in men. However, the paper noted that future studies should include larger sample sizes of men.10 All in all, within current literature, it is clear that there is no consensus when it comes toTXA and men.
TXA is effective at treating melasma. One randomized controlled trial compared 20 patients taking 250 mg TXA twice a day and 17 patients receiving the placebo for 12 weeks.They found that melasma improved in 50% of the patients in the experimental group versus only 5.9% in the placebo group.11 Another trial compared a group of 18 taking the same dosage as the previous study, with 21 patients taking the placebo. Only the group taking TXA saw significant improvement.12
TXA also has a well-established safety profile. Previous studies have reported adverse side effects associated with TXA, however, we must note that these negative side effects occurred at significantly higher doses than what our findings suggest as effective for treating melasma. The most commonly reported side effects include menstrual changes, nausea, gastritis, back pain, and headaches.11 In a controlled study of patients with melasma, a dosage of 250 mg of oral TXA given twice daily for 3 months revealed no serious adverse side effects. There were also no statistically significant differences in side effects between the TXA group and placebo group.12 Within our practice, with over 1500 prescriptions (not including refills) of oral tranexamic acid written, we have only seen two cases of venous thrombosis with extenuating circumstances that exacerbated the possible side effects.
Overall, TXA taken orally at 650 mg daily provides patients with a significant reduction of their melasma. Research on this subject heavily focuses on female subjects and a majority of sample sizes make up females in these studies. While melasma predominantly affects women, it is important to acknowledge the impact that it has on men as well. Tranexamic acid has shown to be efficacious in our male melasma patients, and we hope that more men are offered this treatment method to target their melasma. As research continues to explore the applications of tranexamic acid, practitioners should consider the addition of oral TXA into the treatment plans for male melasma patients, as it has shown to be safe and seems effective based on our experience.
DISCLOSURES
The authors have no disclosures or conflicts.
REFERENCES
1. Callender SLT, Warner GT, Cope E. Treatment of menorrhagia with tranexamic acid. a double- blind trial. Br Med J. 1970;4(5729):214-216. doi:10.1136/bmj.4.5729.214
2. Handel AC, Miot LDB, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89(5):771-782. doi:10.1590/abd1806-4841.20143063
3. Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2018;44(6):814-825. doi:10.1097/ DSS.0000000000001518
4. Cho YH, Park JE, Lim DS, et al. Tranexamic acid inhibits melanogenesis by activating the autophagy system in cultured melanoma cells. J Dermatol Sci. 2017;88(1):96-102. doi:10.1016/j.jdermsci.2017.05.019
5. Jiang J, Akinseye O, Tovar-Garza A, et al. The effect of melasma on self-esteem: A pilot study. Int J Womens Dermatol. 2017;4(1):38-42. doi:10.1016/j.ijwd.2017.11.003
6. Basit H, Godse KV, Al Aboud AM. Melasma. In: StatPearls. StatPearls Publishing; 2023. http://www.ncbi.nlm.nih.gov/books/NBK459271/. Accessed July 10, 2023.
7. Maeda K. Mechanism of action of topical tranexamic acid in the treatment of melasma and sun-induced skin hyperpigmentation. Cosmetics. 2022;9(5):108. doi:10.3390/ cosmetics9050108
8. Okholm SH, Krog J, Hvas AM. Tranexamic acid and its potential anti-inflammatory effect: a systematic review. SeminThromb Hemost. 2022;48(5):568-595. doi:10.1055/s-0042-1742741
9. Lee HC, Thng TGS, Goh CL. Oral tranexamic acid (TA) in the treatment of melasma: A retrospective analysis. J Am Acad Dermatol. 2016;75(2):385-392. doi:10.1016/j.
jaad.2016.03.001
10. Secher JJ, Sidelmann JJ, Ingerslev J, et al. The effect of tranexamic acid and gender on
intraoperative bleeding in orthognathic surgery-a randomized controlled trial. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg. 2018;76(6):1327-1333. doi:10.1016/j. joms.2017.11.015
11. Colferai MMT, Miquelin GM, Steiner D. Evaluation of oral tranexamic acid in the treatment of melasma. J Cosmet Dermatol. 2019;18(5):1495-1501. doi:10.1111/jocd.12830
12. Del Rosario E, Florez-Pollack S, Zapata L, et al. Randomized, placebo-controlled, double-blind study of oral tranexamic acid in the treatment of moderate-to-severe melasma. J Am Acad Dermatol. 2018;78(2):363-369. doi:10.1016/j.jaad.2017.09.053